Near-Infrared Absorbing Chromophores for Medicine and Solar Energy Utilization
Molecular frameworks that absorb light in the near-infrared (NIR) region of the spectrum (NIR-I: 700–1000 nm; NIR-II: 1000–1700 nm) have considerable interest in applications related to medicine due to NIR light penetration through soft tissue for imaging and therapeutic applications. Molecules of this type are also of interest for photo-mimetic energy conversion approaches and can take advantage of the solar flux of NIR photons. Reduced porphyrin macrocycles in particular (i.e. chlorins, bacteriochlorins etc.) exhibit large extinction coefficients in this region, making them an important synthetic target.
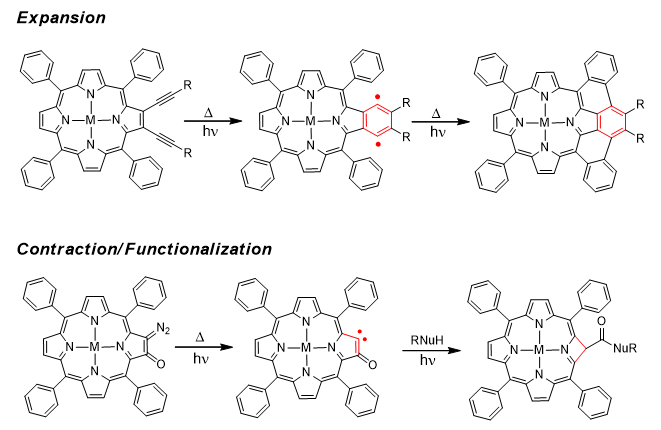 Figure 1. Radical-generating
Figure 1. Radical-generating
porphyrin/chlorin architectures for ring
expansion/contraction functionalization strategies.
Radical-Generating Alkynylporphyrins Cyclization for π-Extension. The synthesis of periphery-modified ring contracted/expanded porphyrin architectures (Figure 1) has long been an area of importance in light of the significant efforts in developing optoelectronic devices, photovoltaic materials, and photoinduced energy or charge transport in dye-sensitized solar energy cells. Fused-ring porphyrins have large molar extinctions, and absorb well into the red and infrared regions of the electromagnetic spectrum. Moreover, the synthesis of fused, benzannulated units via radical cascade reactions of polyacetylenes has been shown to be an effective and accessible pathway towards new aromatic molecules. Within these themes, over the past 24 months our research program has developed synthetic methods to prepared di- and tetraalkynylporphyrins and control their thermal and photochemical reactivities to yield a suite of unique p-extended frameworks. More importantly, in parallel we have investigated their mechanisms and elucidated experimentally and computationally why these reactions behave as they do and learned how to control their outcomes.
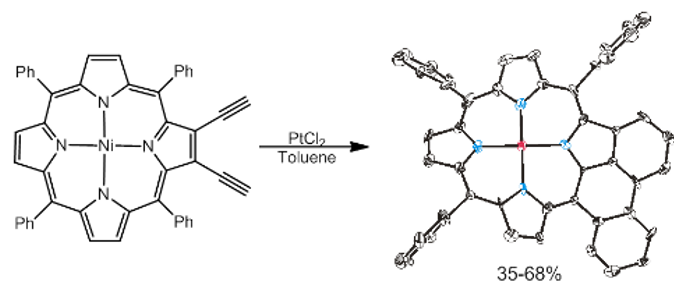 Figure 2. Pt-catalyzed cyclization
Figure 2. Pt-catalyzed cyclization
of dialkynylporphyrins to form
phenanthroporphyrin products.
Our larger contribution in this area involves using Bergman cyclization to activate trans diradical formation and consequential ring-addition across the adjacent meso-phenyl rings. Additional alkyne functionalities create the potential for multiple radical reactions, which are indeed thermodynamically plausible by DFT analysis, but the electronic energy loss via steric pyrrole puckering creates kinetic barriers to the double cyclization events that hinder yields and lead to side reactions. However, use of catalytic quantities of Pt(II) (0.1-1.0 equiv) can be applied to overcome this problem and generate high yield (~70%), phenanthroporphyrin cyclized products (Figure 2) at modest temperature (as low as 40 °C, 35%).
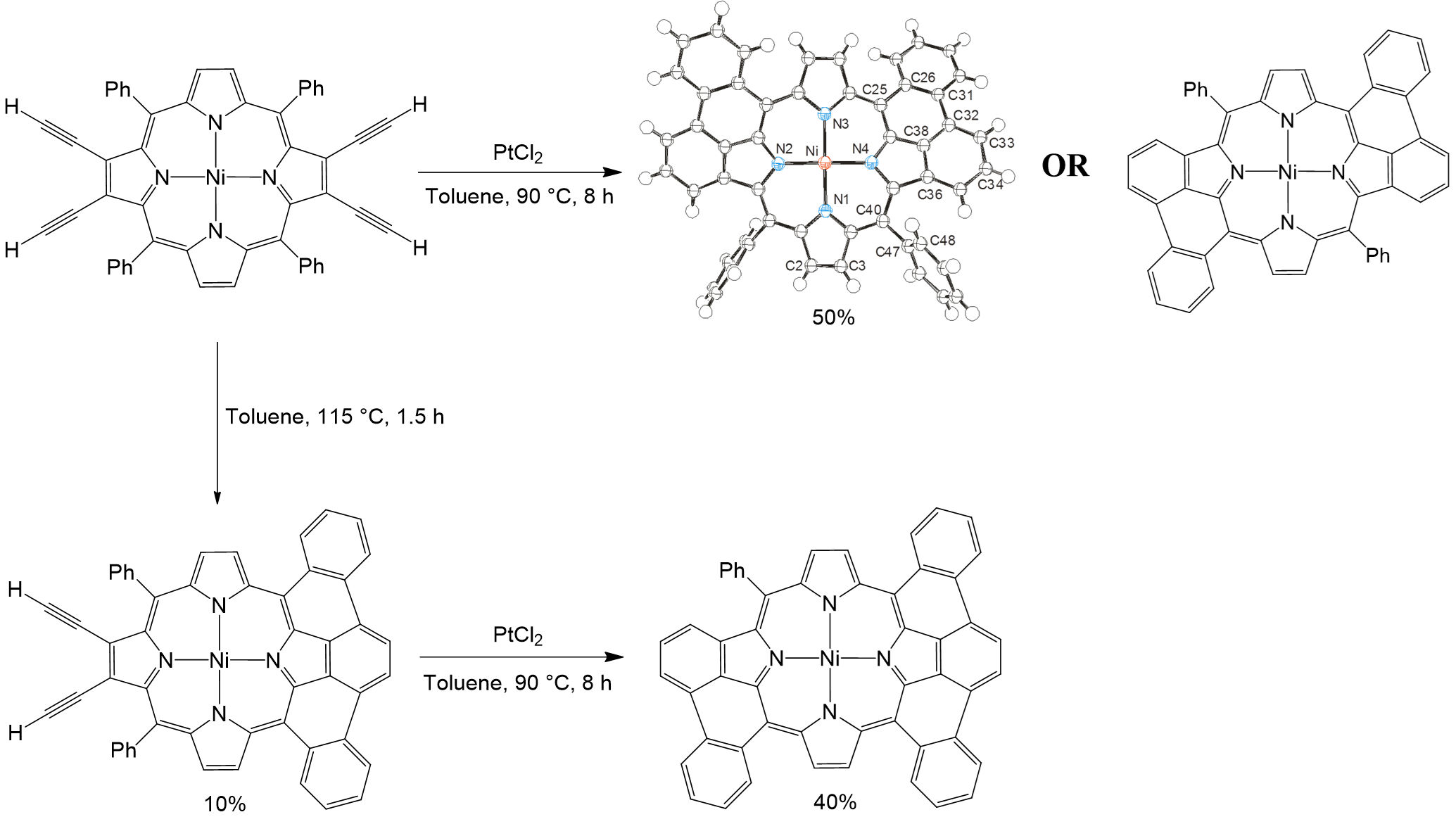 Figure 3. Pt-catalyzed
Figure 3. Pt-catalyzed
formation of bis(phenanthro)- and
piceno-phenanthroporphyrin products.
When this strategy is applied to the more sterically encumbered tetraalkynylporphyrins (R=H) at 90 °C, the reaction affords the unprecedented formation of 50% mixture of the syn and anti bis(phenanthro)porphyrin product, (Figure 3). Alternatively, the tetraalkynylporphryin can be thermally cyclized to generate the picenoporphyrin product, and subsequently reacted with PtCl2 to form the unique piceno-phenanthroporphyrin in 40% yield. These two reactions provide controlled routes to highly unsual and asymmetric p-extended porphyrin products. Moreover, the phenanthroporphyrin structure also indicates that a Pt-C bond is formed at one of the formally radical carbon positons, further elucidating the mechanistic route to this cyclization event.
Photochemically, we have also worked diligently to understand the optically-induced Bergman cyclization mechanism by which these compounds form picenoporphyrin products. Computational and small molecule approahes over the past decade have suggested that the first excited singlet state may be the surface on which Bergman cyclization occurs upon UV-excitation. Since these alkynylporphyrins can be photocyclized with longer wavelengths in the visible spectra region, it hasn’t been clear whether the initially excited 1ππ* or the much longer-lived 3ππ* state is responsible for this outcome. To test this hypothesis, we took advantage of the established short 1ππ* lifetime of Pt(II) porphyrins (<15 ps) and long-lived 3ππ* states to photochemically activate this derivative with electronic preference. Historically, 1ππ* excitation is presumed to lead to Bergman product while 3ππ* photosensitization mainly produces alkyne-reduced species since 3ππ* population promotes out-of-plane distortions about the double bond for simple enediynes, as well as in-plane distortions at the terminal carbon for aromatic enediyne systems. Our frameworks are perfect architectures on which to test the excited state dynamic route of picenoporphyrin product formation by Bergman cyclization because the steric hindrance of the meso-phenyl rings and rapid internal quenching reaction discriminate against bimolecular, 3ππ*-like side reactions, while allowing picenoporphyrin products to readily form. Indeed, the thermal Bergman cyclization of the Pt(II) derivative proceeds in high yield, and at reduced temperature (>20 °C) relative to the Zn(II) and free base analogues (Figure 4).
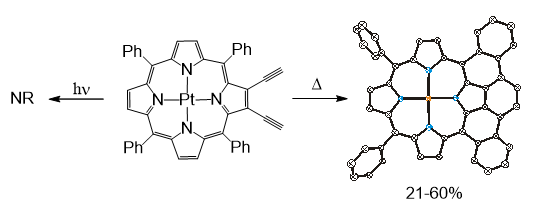 Figure 4. Thermal Bergman cyclization
Figure 4. Thermal Bergman cyclization
of Pt(II) dialkynylporphyrins is facile whereas
triplet-state population leads to no reaction.
However, photochemical population of the 3ππ* state reveals no photoproduct formation of any kind (96% starting material recovery) indicating that Bergman cyclization formally proceeds along the singlet surface, even in the excited state. Thus, the thermal and photoreactions are truly spin-state controlled and in this manner, completely orthogonal reaction trajectories. We are currently using ultrafast transient IR measurements to kinetically visualize bond rearrangements.
Related Publications
- Expansion and Contraction: Shaping the Porphyrin Boundary via Diradical Reactivity, Leigh J.K. Boerner, David F Dye, Tillmann Köpke, Jeffrey M. Zaleski, Coord. Chem. Rev., (2013), 257(2), 599-620.
- Spin-state Control of Thermal and Photochemical Bergman Cyclization, Leigh J.K. Boerner, Maren Pink, Hyunsoo Park, Amanda LeSueur, Jeffrey M. Zaleski, Chem. Comm., (2013), 49, 2145-2147.
- Synthesis of Unique Extended pi-Structures by Pt-Mediated Benzannulation of Nickel(II) Tetraalkynylporphyrins, Leigh J. K. Boerner, Mahendra Nath, Maren Pink, Jeffrey M. Zaleski, Chem. Eur. J., (2011), 17(34), 9311-9315.
- PtCl2 Catalyzed Benzannulation of Nickel(II) 2,3-dialkynylporphyrins to Form Unusual Phenanthroporphyrins, Mahendra Nath, Maren Pink, Jeffrey M. Zaleski, J. Organometallic Chem., (2011), 696(25), 4152-4157.
- Synthesis of Unique Extended -Structures by Pt-Mediated Benzannulation of Nickel(II) Tetraalkynylporphyrins, Leigh J. K. Boerner, Mahendra Nath, Maren Pink, Jeffrey M. Zaleski, Chem. Eur. J., (2011), 17(34), 9311-9315.
- Gating the Mechanistic Pathway to the Elusive 4-Membered Ring Azeteoporphyrin, David F. Dye, Tillmann Köpke, Raghunath Ozhapakkam Ramabhadran, Krishman Raghavachari*, and Jeffrey M. Zaleski*, J. Am. Chem. Soc., (2011), 133(33), 13110-13120.
- Efficient Silver-Mediated Acetalation of ,-Functionalized Chlorins, Tillmann Köpke, Maren Pink, and Jeffrey M. Zaleski, Synlett, (2008), 12, 1882-1888.
- Diazo-containing Molecular Constructs as Potential Anticancer Agents: from Diazo[b]fluorene Natural Products to Photoactivatable Diazo-oxochlorins Tillmann Köpke and Jeffrey M. Zaleski*, Anti-Cancer Agents in Medicinal Chemistry (2008), 8(3), 292-304.
- Electronic and Vibrational Analysis of Porphyrazine Liquid-Crystalline Structure: Toward Photochemical Phase Switching, David F. Dye, Krishnan Raghavachari, and Jeffrey M. Zaleski, Inorg. Chim. Acta, (2008), 361, 1177-1186.
- Expansion by Contraction: Diversifying the Photochemical Reactivity Scope of Diazo-oxochlorins Toward Development of In Situ Alkylating Agents, Tillmann Köpke, Maren Pink, and Jeffrey M. Zaleski, J. Am. Chem. Soc., (2008), 130(47) 15864-15871.
- Elucidation of the Extraordinary 4-membered Pyrrole Ring-contracted Azeteoporphyrinoid as an intermediate in Chlorin Oxidation, Tillmann Köpke, Maren Pink, and Jeffrey M. Zaleski, Chem. Commun., (2006), 4940-4942.
- Photochemical Preparation of Pyrrole Ring-contracted Chlorins by the Wolff Rearrangement, Tillmann Köpke, Maren Pink, and Jeffrey M. Zaleski, Org. Biomol. Chem., (2006), 4, 4059-4062. (Issue Cover)
- A Facile Synthesis of 2-Diazo-3-Oxochlorins by Lewis Acid Activation: Selective Modification of -Electron Conjugated Macrocycles, Tillmann Köpke, Maren Pink, and Jeffrey M. Zaleski, Synlett, (2006), 14, 2183-2186.